Abstract
Background
Hematopoietic stem cell transplant (HCT) is the only established curative approach for sickle cell disease (SCD). Due to the high toxicity profile of myeloablative regimens, some adult patients with severe sickle cell anemia are not eligible for such therapy. However, a new adult nonmyeloablative protocol (National Institutes of Health, NIH protocol) described by Hsieh et al. (2014) was able to achieve a curative degree of mixed donor chimerisms with minimal transplant-related complications. The NIH protocol includes alemtuzumab, low dose total-body irradiation (TBI) of 300 cGy, and prolonged sirolimus for graft-versus-host disease (GVHD) prophylaxis for those recipients with a matched-sibling donor (MSD).
The Alberta Children's Hospital has adopted this published regimen for MSD HCT in adolescents and younger children with SCD since the NIH data was presented in 2013.To our knowledge, there is no published literature describing the application of the NIH protocol in a pediatric population; the youngest subject in the referenced adult study was 16 years of age.
Methods
This retrospective cohort describes outcomes of nonmyeloablative allogeneic MSD HCT in children and adolescents with SCD who underwent HCT between 2013-2017. Fourteen patients with SCD under 18 years of age at the time of HCT received the NIH conditioning regimen. HLA-matched siblings had either normal hemoglobin or sickle cell trait and of the appropriate weight/height to donate peripheral blood stem cells (PBSCs) without blood priming of the apheresis circuit.
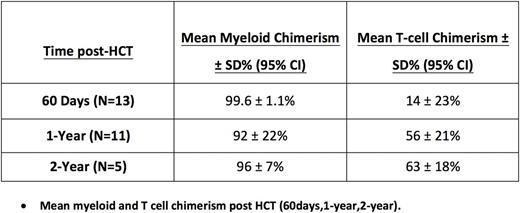
The conditioning regimen consisted of alemtuzumab 1.0 mg/kg administered subcutaneously daily divided over five days (Days -7 to Day -3). Patients received a single TBI dose of 300 cGy on day -2, with testicular shielding for male recipients. GVHD prophylaxis consisted of a sirolimus loading dose of 3mg/m2/dose (PO) on day -1, followed by 1mg/m2/dose once daily starting on Day 0. PBSCs were typically collected on Day -3 to allow for cancellation of the HCT before TBI in the event of failed mobilization. Unmanipulated PBSCs were infused fresh on Day 0. Patients were eligible for early discharge post-transplant even prior to neutrophil engraftment. Weaning of sirolimus was initiated no earlier than 1-year post-HCT and if donor T-cell chimerisms were greater than 50%. Treatment failure was defined as clinical manifestation of SCD, failure to start weaning sirolimus by 2-years post-HSCT, or a failure to complete a sirolimus wean by 2.5 years post-HCT.
Results
Fourteen patients (3 M,11 F) are included on this retrospective study. Median age at HCT was 11 years. Post-HCT follow-up ranges from 1 to 48 months (median 17). There were no failed donor stem cell mobilizations. Patients received a median of 11.4x106 CD34 cells per kilogram of body weight.
All patients were discharged home prior to neutrophil engraftment with a median hospital stay of 7 days (range 5-19). All patients had donor neutrophil engraftment at a median of 21 days (mean= 22, SD= 4) and platelet engraftment at median of 14 days (mean= 12, SD= 14). Six patients never required platelet transfusion. Readmission after initial discharge was required in 9/14 patients with an average of 1 readmission/patient (range 1-4), typically for fever or CMV pre-emptive therapy. One patient developed septic shock with Acinetobacter ursingii related to poor central line care at home.
All patients are currently alive. Post-HCT, there have been no cases of graft failure, sickling crises, veno-occlusive disease, idiopathic pneumonia syndrome, cerebral hemorrhage, PRES, or PTLD. Although 6/14 patients had CMV reactivation, only 3 patients required pre-emptive therapy. None had CMV/EBV disease.
There were no treatment failures as defined by protocol. Currently, 8 patients are off sirolimus, 1 is currently weaning, with no secondary graft failure events. Only 2 patients over 1-year post-HCT have not initiated sirolimus weaning. There have been no cases of acute or chronic graft-versus-host disease.
Conclusions
Nonmyeloablative conditioning regimen is safe and effective as curative therapy for children with SCD. Outpatient management pre-engraftment was feasible in this population. In contrast to adult data, sirolimus weaning was possible in most patients by one-year post-HCT. Graft integrity was maintained after discontinuation of sirolimus in all eligible patients.
Guilcher: Patient Access Solutions: Consultancy; Canada Market Research: Consultancy; Jazz pharmaceuticals: Consultancy. Bruce: Bristol-Meyers-Squibb - consultant 2016-2017: Consultancy; ovartis consult and ad board 2015-2016: Consultancy; Apopharma 2015: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal